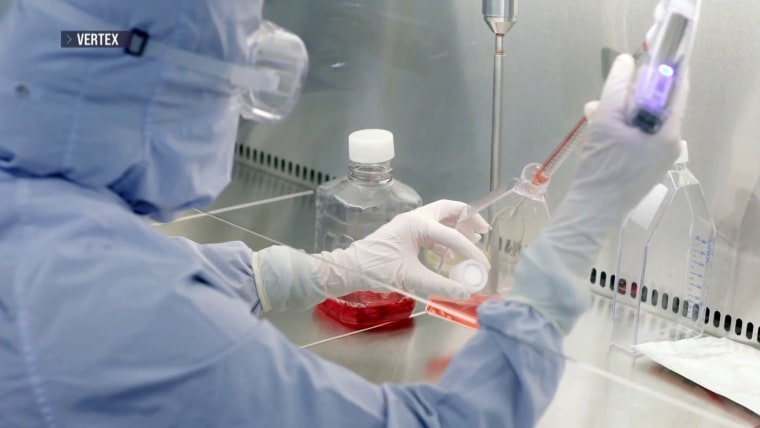
The Food and Drug Administration granted approval for a potent treatment for sickle cell disease, an incurable ailment that impacts over 100,000 Americans, with a disproportionately African American population.
Casgevy, a treatment developed in collaboration with CRISPR Therapeutics and Vertex Pharmaceuticals, is the inaugural pharmaceutical product to receive approval in the United States that employs the gene-editing tool CRISPR, the inventors of which were awarded the Nobel Prize in chemistry in 2020.
Dr. Alexis Thompson, chief of the division of hematology at Children’s Hospital of Philadelphia and a former consultant for Vertex, opined that “this is a turning point in the field.” “It is truly remarkable how quickly we went from the Nobel Prize being awarded for the discovery of CRISPR to seeing it become an officially approved product.”
The sanction signifies the initial of two prospective advancements concerning the hereditary blood disorder. Bluebird Bio’s Lyfgenia, a gene therapy, was granted approval by the FDA on Friday as an additional treatment option for sickle cell disease. Both therapies function through the genetic modification of the stem cells of the patient.
Up until now, the sole established remedy for sickle cell disease was a bone marrow transplant from a donor, an operation that entailed the formidable tasks of locating a compatible donor and carried the risk of immune system rejection.
Approved for individuals 12 years and older, Casgevy eliminates the requirement for a donor. By modifying the DNA present in a patient’s stem cells with CRISPR, the disease-causing gene is eliminated.
“The patient acts as their own donor,” stated Thompson.
Asmaa Ferdjallah, a pediatric hematologist and specialist in bone marrow transplantation at the Mayo Clinic in Rochester, Minnesota, characterized it as a “game-changer.” “With this news, it is sufficient to reimagine and re-discuss sickle cell disease as a curable condition, rather than as a chronic, excruciating, and debilitating illness.”
Vertex stated that the new therapy is still prohibitively costly, at $2.2 million per patient. According to experts, the pricing strategy might render it financially unattainable for a significant number of families. Additionally, the cost of care associated with the treatment, such as hospitalization or chemotherapy, is not included in that price.
According to Dr. Rabi Hanna, a pediatric hematologist-oncologist at the Cleveland Clinic and a former member of Vertex’s advisory board, “We must ensure that it is easily accessible.” “This could be an equalizer for sickle cell patients, as the disease prevents many from pursuing career opportunities.”
“Families have had knowledge of this since the earliest stages of research, and they have patiently awaited it for years,” Ferdjallah explained. “It has been eagerly anticipated not only by families and patients, but also by medical professionals and practitioners.”
How Casgevy functions
Sickle cell disease causes red blood cells, which are ordinarily disk-shaped, to assume the form of a sickle or crescent. This alteration may result in cellular aggregates, which may obstruct blood vessels and coagulate, depriving tissues of oxygen. Patients may suffer from severe agony, difficulty breathing, or a stroke.
Casgevy functions by modifying the DNA of a patient’s stem cells, which are accountable for the production of blood cells, in order to eradicate the production of sickle-shaped cells.
Although the procedure may appear to be a singular occurrence, it entails several stages that accumulate over several months prior to the patient receiving the modified stem cells. According to Hanna, the process commences with a sequence of blood transfusions spanning a duration of three to four months. Subsequently, stem cells are isolated from the bone marrow of the patient and transported to a laboratory for editing.
However, prior to reinfusion into the patient, physicians must ascertain that the body is devoid of any compromised stem cells. Chemotherapy is utilized to accomplish this by destroying the patient’s bone marrow.
Subsequently, the modified stem cells may be reinfused into the patient, after which the patient must remain in the hospital for an additional fortnight to facilitate cell proliferation and recovery.
Hanna stated that he is “cautious” at all times when disclosing the one-time treatment to patients and their families, as they might develop unattainable expectations.
He stated, “There are several stages to this journey.”
In the clinical trial, thirty of the forty-six participants from the United States and abroad received follow-up care for a minimum of eighteen months following treatment. In 29 cases, the treatment has proven to be effective.
Strongly recommended
The CDC issues a warning to visitors to Mexico regarding Rocky Mountain spotted fever.
Phoenix resident LaRae Morning, age 29, was one of the trial participants who benefited from the treatment.
Her physicians predicted she would not survive beyond the age of 11. Due to her mother’s frequent hospital visits during Morning’s childhood and adolescence, her mother lost multiple jobs.
Morning initially had second thoughts about enrolling in the clinical trial at Sarah Cannon Research Institute and HCA Healthcare’s The Children’s Hospital at TriStar Centennial in Nashville, Tennessee, which she did in April 2021.
For treatment, she was required to fly to Nashville once per month from Phoenix. It involved a series of eight-hour-long blood transfusions and the administration of plerixafor, a medication that she distinctly remembered inducing her severe abdominal cramps.
Her epidermis changed color and hair began to fall out as soon as she began chemotherapy, giving her appearance similar to that of a chemical burn. She experienced nausea as well.
Six to seven months passed before she regained her normal senses after undergoing the CRISPR treatment. She currently attends coffee shops, spends time with her friends, and completes the first semester of law school in Washington, D.C., and she claims to be experiencing the benefits.
She remarked of the trial, “I am ecstatic that I carried it through to this point.” “As ordinary as the average person.” When I awake, I run a 5K. I engage in weightlifting. I am able to swim if I so desired. “I am still uncertain as to how far I can stretch it, or in other words, what am I able to do with it.”
Several other trial participants have had the same experience, according to Dr. Monica Bhatia, chief of pediatric stem cell transplantation at Irving Medical Center, New York Presbyterian/Columbia University. Bhatia is a principal investigator at one of the New York City clinical trial sites.
After about three to four months, the majority of patients resumed school, the gym, or other activities—”things that a lot of people take for granted,” she explained.
Bhatia stated, “They are truly capable of living life without restrictions.”
The medical director of pediatric hematology-oncology at the Sarah Cannon Research Institute, Dr. Haydar Frangoul, expressed optimism that the treatment will offer solace to a greater number of patients.
“This is a monumental moment for patients with sickle cell disease,” said Frangoul, who treated Morning and served as the clinical trial’s principal investigator.
Extended-term inquiries
While the efficacy of Casgevy has been demonstrated, there remains uncertainty regarding potential long-term consequences due to the two-year duration of the clinical trial.
An FDA advisory committee deliberated at a meeting in October on the potential for “off-target” effects, wherein the gene-editing tool inadvertently deletes segments of DNA apart from the intended target. The committee also explored how the FDA ought to approach this risk in the future.
Thompson stated that while it is unknown what impacts an off-target edit would have on a patient, there is concern that it may lead to unintended health consequences in the future. “There do not appear to be any quantifiable consequences as of this moment.”
However, the FDA added the most severe safety warning label, a boxed warning, to Lyfgenia by Bluebird Bio, noting that the treatment can cause uncommon cases of certain blood cancers.
The FDA’s Center for Biologics Evaluation and Research’s Director of the Office of Therapeutic Products, Dr. Nicole Verdun, stated that the warning was issued for Lyfgenia after two patients who received the therapy in a clinical trial perished of a form of leukemia.
The causal relationship between the cancer and the gene therapy or another component of the treatment regimen, such as chemotherapy, remains uncertain. However, Verdun expressed the agency’s belief that the fatalities “elevated to the level of a black-box warning.” She stated that no cases were observed during the Vertex clinical trial.
Read Also: 8 Everyday Habits That Can Damage Our Health
As part of a 15-year post-approval study for Casgevy, Bhatia will monitor the patients for indications of persistent side effects.
She stated, “Long-term follow-up will continue to be vital.”
Vega, Christopher, is hospitalized.
Christopher Vega, age 31, of Allentown, Pennsylvania, on the day he received his CRISPR-edited stem cell infusion on December 7, 2020.Vega, Christopher (Credit).
Christopher Vega, age 31, from Allentown, Pennsylvania, stated that he is content to be free from chronic pain and is not concerned about the possibility of long-term consequences.
In late 2020, he began participating in the clinical trial at the Children’s Hospital of Philadelphia. Ever since his early childhood, he had been afflicted with chronic exhaustion, which resulted in an annual hospitalization due to a severe pain crisis.
“Sometimes things occur for a purpose, my mother would always tell me when I was younger. “And I had such difficulty believing that because I was constantly asking myself, ‘Why me?'” he continued.
Vega stated that despite the fact that the treatment was not always straightforward (he experienced temporary hair loss, nausea, and weakness, and developed skin lesions), it was well worth it.
Vega, who is currently caring for his 5-year-old daughter while attending the Los Angeles Film School online for visual effects, stated, “I am an entirely different person.”
“I would experience brief moments of anxiety that I was approaching a crisis,” he explained. “However, after years of deliberation, I am gradually accepting the fact that everything is fine and that I will be physically present.”